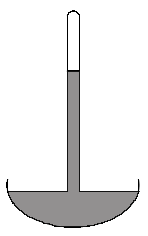
(a) The figure below shows a mercury barometer. At atmospheric pressure, the length of the mercury is 76.0 cm. (Density of mercury is 13.6 g/cm3)
(i) Describe how the barometer can be used to measure atmospheric pressure.
(ii) Hence, calculate the value of atmospheric pressure in pascal.
(iii) Explain why water is not a suitable liquid to be used in the barometer.
(b) The figure below shows a design of a hydraulic brake system. The pedal is pushing at the small piston.
(i) Explain how the force at the pedal can be transmitted to the large piston.
(ii) Hence calculate the force on the large piston when the force on the small piston is 110N.
(iii) Suggest why water is not suitable to be used as a brake fluid.
*************************
Answer:
(a)
(i) The pressure due to the length of the mercury column above the mercury level in the bowl is balanced by the atmospheric pressure. Hence, by measuring the pressure due to the mercury column, we can find the value of the atmospheric pressure.
The equation for the atmospheric pressure is given by P = hρg
(ii) Density of mercury = 13.6 g/cm3 = 13600 kg/m3
Hence, P = hρg = 0.76 * 13600 * 10 = 1.03 * 105 Pa.
(iii) Since the density of water is lower than mercury, the height of the water column will be larger (about 10m) given that atmospheric pressure is constant. The size of the barometer would be too large.
(b)
(i) Since the brake fluid is incompressible, pressure at the small piston is transferred to the large piston. Thus, force is transmitted from the pedal (at the small piston) to the large piston in this manner.
(ii) Since P1 (at the small piston) = P2 (at the large piston)
F1/A1 = F2/A2
110/0.00070 = F2/0.0037
F2 = 581 N
(iii) Brake fluid is better because it can reduce corrosion in the hydraulic system. It can also act as a lubricant for the moving parts in the system.