RI 1998 Sec 3 EOY P2 Q5
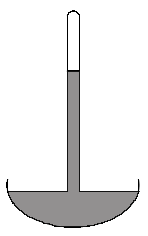
(a) Draw a labelled diagram of a simple mercury barometer.
State and indicate clearly on your diagram how such a barometer may be used to obtain a value for atmospheric pressure.
(b) A marine expedition makes a survey of an ocean floor which is 0.50 km below sea-level. Assuming that the density of sea-water is 1.0 * 10³ kg/m³, estimate in Pa, the total pressure on the ocean floor.
Answer:
(a)
How to use barometer:
Place the barometer where the atmospheric pressure is to be measured. Then measure the height of the mercury column from the highest level of the mercury in the tube to the mercury level at the top of the tub, as shown in the figure.
By taking hρg, where h is the height of the mercury column, g is the gravitational value, and ρ is the density of mercury, we can find the atmospheric pressure.
(b) 0.50 km = 500 m
Total pressure = a.t.m. + hρg
= 1.0 * 105 Pa + (500 * 1.0 * 105 * 10) Pa
= 1.0 * 105 Pa + 50 * 106 Pa
= 5.1 * 106 Pa